Sep 24, 2021
Transcript
[RADIOLAB INTRO]
LATIF NASSER: I'm Latif Nasser. This is Radiolab. Today, a story about how we humans rearrange the elemental stuff all around us, and how one man's pursuit of a basic truth about the Earth revealed in an extraterrestrial explosion, a 2,000-year-old Peruvian skeleton, even a strand of his lab assistant's hair, changed the very air we breathe. It comes to us from reporter Avir Mitra.
AVIR MITRA: So I guess we'll start it ...
[ARCHIVE CLIP, interviewer: Okay, good afternoon, Dr. Patterson. It's a pleasure to talk to you.]
AVIR: ... with this guy Clair Patterson. He goes by the name Pat.
[ARCHIVE CLIP, interviewer: And I think we'd like to start this interview with you just telling us a little bit about your background.]
AVIR: This is from an oral history from 1995.
[ARCHIVE CLIP, Clair Patterson: All right. Well, I was born in a small town in the middle of Iowa. It was located in the midst of farmland, rolling prairie-type farmland, central Iowa. And this little town, at that school, it was a small school. All the students knew each other for 12 years.]
[ARCHIVE CLIP, interviewer: So you were enrolled in one school for the whole time?]
[ARCHIVE CLIP, Clair Patterson: One school the whole time. People didn't move in and out. It was sort of a tribal interaction.]
AVIR: And so Pat's tribe ...
[ARCHIVE CLIP, Clair Patterson: We learned how to hunt.]
AVIR: ... did all the things you do growing up in the country.
[ARCHIVE CLIP, Clair Patterson: Learned how to swim and fish. And we saw crops being planted. We knew about farm animals.]
LYDIA DENWORTH: But he was—he was ...
AVIR: This is science journalist Lydia Denworth.
LYDIA DENWORTH: He was in some ways a farm boy, but ...
[ARCHIVE CLIP, Clair Patterson: I knew I was different than most—most people.]
LYDIA DENWORTH: He was the kind of kid who asked his mother all kinds of questions about the world. Like ...
[ARCHIVE CLIP, Clair Patterson: Why is a drop of water round?]
LYDIA DENWORTH: Things like that. He set up a chemistry lab in his basement when he was 12.
LATIF: Oh wow!
AVIR: In high school, he, you know, was always like ...
[ARCHIVE CLIP, Clair Patterson: Science teacher would say something about electricity being a fluid.]
AVIR: ... correcting his teachers.
[ARCHIVE CLIP, Clair Patterson: I had to explain to him about electrons, and ...]
LYDIA DENWORTH: He was just always, always fascinated by how the world worked. And he had the ability to see beyond what others saw. You know, he made leaps.
AVIR: So, like, for example, this is a kid from a small town. He goes to a small high school.
LATIF: Yeah.
AVIR: Small college. And graduates, and his first job out of college, he's working ...
[ARCHIVE CLIP, Clair Patterson: On the atomic bomb.]
AVIR: ... for the Manhattan Project.
LATIF: Oh wow.
AVIR: Now to back up a little bit. At the beginning of World War II, around 1940, there were these European scientists.
LYDIA DENWORTH: Like Einstein and others.
AVIR: Who were immigrating to the US and telling anyone who would listen the Germans were making a bomb, an atomic bomb.
LYDIA DENWORTH: And they were pushing the Americans to make sure that they didn't get behind the Germans.
AVIR: And so when Pat graduated, some of his professors pushed him ...
[ARCHIVE CLIP, Clair Patterson: To join the Army.]
LYDIA DENWORTH: And help the American effort to create a nuclear bomb.
AVIR: So Pat enlisted, got shipped down to Oak Ridge, Tennessee.
LYDIA DENWORTH: With his wife Laurie.
AVIR: Laurie Patterson, she was also a scientist. Brilliant chemist, in fact. And also worked on the Manhattan Project. So anyway ...
LYDIA DENWORTH: And Oak Ridge was one of those places that they made a city overnight.
AVIR: 75,000 people in the end were working and living there.
[ARCHIVE CLIP, Clair Patterson: And we had a little dog, and we went back and forth on a bus to work every day.]
AVIR: To these top secret, state of the art laboratories.
[ARCHIVE CLIP, Clair Patterson: Buried in the mountains.]
AVIR: And I don't think he knew what he would be doing when he got there, but he ends up dead smack in the middle of the whole thing.
LATIF: Like, dead smack in the middle of the bomb?
AVIR: Yeah. His job at Oak Ridge was to make the actual stuff in the bomb that goes boom, which was uranium.
LATIF: Hmm.
AVIR: And uranium is an interesting character—an element, really. It's the heaviest element that occurs, naturally at least. So, like, you know, every element is made up of three things: protons, neutrons and electrons.
LATIF: Mm-hmm.
AVIR: Protons and neutrons are in the middle. They're kind of what define the element. So every element has a different amount of these things. And uranium has the most of them. Like, just to give you an example, hydrogen has a weight of one, helium four, carbon has, like, 12. But uranium's out here. It weighs 238. It's just huge.
LATIF: Right.
AVIR: All right? So that makes it—and this is key—unstable. Because the thing you need to do with a nuclear bomb is to break an atom apart. And uranium? Like, I think of uranium like a guy who's walking around with, like, a ton of grocery bags. You know, he's just holding way too many bags.
LATIF: [laughs]
AVIR: You know, walking from the grocery store back to his car, and you know this dude's just not going to be able to make it. Along the way he's gonna drop a bag. And that's basically uranium. It's just too big. It's holding onto too many things. So now if that's just a couple uranium atoms mixed in with other atoms here or there, it's no big deal. But if you can put a thousand uranium dudes in a parking lot overfilled with grocery bags, and if you just, like, threw an apple at one of those guys, or threw a grocery bag at one of those guys, right?
LATIF: Right.
AVIR: That dude's gonna drop the bag. And then that bag is gonna fall and kind of tumble onto the guy next to him. He drops two bags. And then four dudes around him tumble and fall and just drop some more bags.
LATIF: Right.
AVIR: It's gonna set off a chain reaction, basically. And that's where ...
LATIF: Right.
AVIR: ... shit gets nuclear. So Pat's job at Oak Ridge was to use this newfangled machine called the mass spectrometer. And what that does is it isolates different elements in regular rocks. So he's finding uranium in granite rocks. He's pulverizing these rocks, isolating uranium, purifying uranium. And he basically just spent two years doing this. And then August 6, 1945.
[ARCHIVE CLIP, Franklin D. Roosevelt: A short time ago, an American airplane dropped one bomb on Hiroshima, and destroyed its usefulness to the enemy.]
AVIR: The United States dropped the U bomb, the uranium bomb.
[ARCHIVE CLIP, Franklin D. Roosevelt: With this bomb, we have now added a new and revolutionary increase in destruction, the like of which has never been seen on this Earth.]
AVIR: When the bomb exploded, temperatures on the ground reached over 7,000 degrees Fahrenheit. Tens of thousands of people were killed instantly. And that's just from the energy of the explosion. But then what happens is something arguably more horrifying, because when a uranium bomb explodes, now there's all this uranium around just spewing out all these protons and neutrons. And even if you didn't get killed by the bomb, those protons and neutrons they're gonna go right into your body.
[ARCHIVE CLIP: Hospitals filled with patients who had not seemed sick before. At first, they were quarantined, considered victims of a mysterious infectious disease.]
AVIR: And if there's enough of it around, it's literally gonna tear your body apart from the inside.
[ARCHIVE CLIP: They were vomiting, bleeding from the gums and purple spots appeared on their skin. Some could not be touched because their skin slipped off in huge glove-like pieces. These people were special victims of the atomic bomb.]
AVIR: And that's exactly what happened in Hiroshima. And then we dropped a second bomb on Nagasaki.
[ARCHIVE CLIP, Clair Patterson: During the war developed these concepts ...]
AVIR: Pat said, you know, as this 22-year-old kid straight out of college, he never really fully understood the scope of what he was doing. And that these—these mentors of his ...
[ARCHIVE CLIP, Clair Patterson: These mentors, these professor mentors, they knew that they were working as engineers on a hideous weapon of warfare, and they conveyed to young people like me that this is the thing to do. This hideous crime we were committing was—it was a necessary thing.]
AVIR: After Japan surrenders, you know, Pat and his wife were given these commemorative pins that said "Manhattan Project-A Bomb." And according to his wife, they just threw them in the trash.
LATIF: So—so what is that? Like, is that regret or anger or shame? Or, like ...
AVIR: I think it was probably all of those things. I think he felt like this thing that had sort of inspired him and moved him and defined his life, which was basically just like curiosity about the world, understanding things, had kind of been taken advantage of and sort of used to create this real horror. And so he just wanted to get away from all of that. I think he just wants to get back to doing science that's free of all of that.
[ARCHIVE CLIP, Clair Patterson: True science. It's just for understanding for its own end. Yes!]
AVIR: But that's not quite how it worked out for him. So after the bomb project, Pat and his wife Laurie move back to Chicago, and he enrolls to get his chemistry PhD at the university there, the University of Chicago.
[ARCHIVE CLIP, Clair Patterson: Started taking courses.]
AVIR: And one day, this professor comes up to him.
[ARCHIVE CLIP, Clair Patterson: And he said, "Hey, Pat."]
AVIR: "Look, I'm trying to answer a question. How old is this rock that we’re sitting on? Like, how old is the Earth?"
LATIF: We didn't know that?
AVIR: No.
LYDIA DENWORTH: Nobody knew the age of the Earth.
AVIR: People had been trying to guess at this for a long time.
LYDIA DENWORTH: It started with somebody adding up all the ages of everybody in the Bible. [laughs]
AVIR: Which got us to about, like, 6,000 years old.
LYDIA DENWORTH: It's not right. [laughs]
AVIR: And then, you know, people start finding dinosaur fossils, and it's like, okay, well, I guess this has to be a little longer than that. But still no one knew exactly how much longer.
LATIF: Hmm.
[ARCHIVE CLIP, Clair Patterson: Anyway, so that's ...]
AVIR: So Pat's professor says to him, "This is perfect for you, it’s pure chemistry."
[ARCHIVE CLIP, Clair Patterson: And you'll be famous because you measured the age of the Earth.]
[ARCHIVE CLIP, interviewer: And what did you say?]
[ARCHIVE CLIP, Clair Patterson: I said, "Good! I will do that."]
AVIR: And then his professor said to him ...
[ARCHIVE CLIP, Clair Patterson: It'll be duck soup, Patterson.]
AVIR: "It's gonna be duck soup." Which I guess is a way of saying it's easy.
LATIF: That's my—that's my favorite Marx Brothers movie.
AVIR: Duck Soup?
LATIF: Yeah.
AVIR: Hmm.
LATIF: Anyway. Okay, sorry. Keep going.
AVIR: Okay, so Pat's got this job now, right? No making bombs, it's just this pure scientific question. But the crazy thing is, the key to answering this question is the same thing that caused all this horror in the atom bomb.
LATIF: Okay.
AVIR: So ...
LATIF: Intrigued.
AVIR: So basically, uranium is sitting around in all these rocks on Earth doing its thing, right? Over time, it slowly drops a proton or neutron here or there. And since it's just mixed up in other rocks, it's not a huge deal. But while it's not really hurting anyone, it is doing something else: it's decaying. It's actually changing into a different atom, a different element. So over time, really, really slowly, as it spits out protons and neutrons, the uranium atom turns into, like, thorium, then turns into radon, and turns into bismuth. Ultimately, it turns into lead. And lead is stable. So it's just gonna be lead forever, as far as we know.
LATIF: Mm-hmm.
AVIR: And the thing about this decay is that it happens at a very predictable rate. It's very, very slow, but scientists figured out that, like, say if you start out with a rock that only had uranium when it was formed, now if you look at the rock and you see, I don't know, a gram of lead, you know how long it took to make that lead, you could tell how long that rock's been around.
LATIF: Got it.
AVIR: That basically means that you have a super accurate clock hidden inside every rock.
LATIF: Clock rocks.
AVIR: Clock rocks.
LATIF: Got it.
AVIR: But the problem with rocks on Earth when you try to date them, they’re just not good dates. No, I'm just kidding.
LATIF: [laughs]
AVIR: The thing about rocks is they ...
LATIF: People look at you funny, yeah.
AVIR: Yeah, it's just awkward, you know? No, but the problem is that, you know, when you figure out the age of a rock, that's great, but it just tells you the age of that specific rock. It just tells you how old that particular rock is. Like, that's all you really know.
LATIF: Yeah. How do you know that's the oldest rock, or there's another rock that's even older? Or this one actually was just made yesterday in a volcano. Like, who knows?
AVIR: Yeah, exactly. That's the problem. The Earth is just a chaotic place, you know? And so somehow you need to get your hands on a rock that you know for sure formed at the same time as the Earth.
LATIF: [laughs] And how could you possibly do that?
AVIR: Well, what Pat had to do is go to the very center of an explosion that was about a thousand times bigger that the bomb that was dropped on Hiroshima. Which happened thanks to nature, actually. Not humans.
AVIR: Okeedoke.
AVIR: Out in the middle of this desert in northern Arizona. It's like, red rocks, flatlands. All you could see for miles.
AVIR: All right, here's—oh, all right.
AVIR: I actually went there. Met up with this guy.
AVIR: Hello!
MICHAEL SCHWAB: Avir, right?
AVIR: That's right.
MICHAEL SCHWAB: How's it going?
AVIR: Hey.
MICHAEL SCHWAB: Michael.
AVIR: Michael Schwab. He's a tour guide.
MICHAEL SCHWAB: So where do you want to do this? Do you want to go out to—to Picture Rock, all the way out there and do this?
AVIR: Yeah, I think that would be cool. Maybe we could just, like, walk and you could teach me.
MICHAEL SCHWAB: Yeah, absolutely. Follow me.
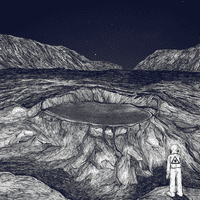
AVIR: So yeah, he walks me on this path. And we're walking and talking until we get to the edge of this ridgeline.
AVIR: Oh, my God!
AVIR: And looked down into this huge crater.
MICHAEL SCHWAB: Just imagine the biggest hole that you could ever possibly grasp, and then just amplify that.
AVIR: I don't know how to describe this, because it really just looks like someone set off a bomb in the middle. And it's just a perfect circle.
MICHAEL SCHWAB: Almost perfect circle, yeah. It's difficult to describe in words.
AVIR: And basically, it's this huge crater in the middle of the desert that's almost a mile in diameter. And it is ...
MICHAEL SCHWAB: If you put the Washington Monument down there at the bottom ...
AVIR: ... deep.
MICHAEL SCHWAB: ... we'd actually be eye level with it.
AVIR: No, you gotta be kidding me.
MICHAEL SCHWAB: Most people when they come out here don't even know what to do with themselves. They just sit in awe of it.
AVIR: Actually went out, talked to some people who were out there.
MAN #1: Big hole, right?
AVIR: [laughs]
MAN #2: It's kind of messing with my head a little bit.
WOMAN #1: It's scary.
MAN #3: Fascinating.
WOMAN #2: A lot of people can determine which way it got here, but I see it as the Majesty of God's hand.
AVIR: It is kind of a miracle how this crater got here. But to explain, a couple years before Patterson was going to answer this age of the Earth question, astrophysicists had strong enough telescopes that they were able to look into the heavens and see how other solar systems had formed. And what they were seeing is that solar systems form at the same time.
LATIF: Forms at the same time as what? As ...?
AVIR: In other words, like, with our solar system you might think oh, Jupiter formed first and then maybe Earth formed later.
LATIF: Right.
AVIR: But no. They all happened together.
LATIF: Got it.
AVIR: But one planet that should have become a planet, for whatever reason, didn't clump together right. And it formed the asteroid belt.
LATIF: Hmm.
AVIR: And in that belt, there was this big rock. About, like, 150 feet long, half the size of a football field. And for countless years it was just hanging out, floating in space. So it's just vacuum sealed in space, not getting changed. And then one day, another rock very gently tapped it. And that set it very slightly off of its course. And year after year after year, its orbit is now bringing it ever so closer and closer to the sun.
LATIF: Hmm.
AVIR: And eventually, 50,000 years ago—Mike likes to imagine it was early summer ...
MICHAEL SCHWAB: Yeah, I like to say July 4, this thing hits. It's the best firework show ever.
AVIR: So, July 4, 48,000 BC.
MICHAEL SCHWAB: Ice age.
AVIR: This whole desert was grassland. And people hadn't even crossed the Bering Strait to get over here yet.
MICHAEL SCHWAB: Right. So you had creatures like Melly, the melancholy mammoth, Sid the sloth.
AVIR: And that day, had they looked up, they would have seen this huge flaming rock.
MICHAEL SCHWAB: It would have looked like a great ball of—almost like a bit of the sun came down.
AVIR: Hurtling towards them very fast.
MICHAEL SCHWAB: About 26,000 miles per hour fast. And then next thing you knew ...
AVIR: This flaming rock came falling out of the sky, slammed into Earth in a huge fiery explosion.
[ARCHIVE CLIP: It took just 10 seconds, and Meteor Crater was formed.]
AVIR: Now when this meteor struck, there was so much force that the meteor itself was just like, disintegrated. Most of it disintegrated. But ...
MICHAEL SCHWAB: In here we have a picture of the crater.
AVIR: ... some of it survived the impact.
MICHAEL SCHWAB: But we also have front and center, the largest fragment of meteorite we've recovered from this site.
AVIR: Wow!
MICHAEL SCHWAB: And you're welcome to touch it as well.
AVIR: Whoa, okay, yes. You were not lying. This is—all right, how big is this thing, like, in terms of size?
MICHAEL SCHWAB: This thing is about three feet in length. So the first thing you'll actually notice is that it's not totally smooth.
AVIR: It looks like Swiss cheese.
MICHAEL SCHWAB: It looks like Swiss cheese.
AVIR: Kind of looks like an arrowhead type of shape.
MICHAEL SCHWAB: Or as I'm looking at it now, it kind of looks like a lopsided dinosaur skull.
AVIR: Yeah, it does look like a dinosaur skull.
MICHAEL SCHWAB: But arguably my favorite thing is that people worry all the time, like, "Oh, doesn't anybody try to steal this?"
AVIR: Right. Because I'm considering it.
MICHAEL SCHWAB: If you tried to pick it up and somehow you bring it back to your car, you can have it. Because it weighs 1,406 pounds.
AVIR: This three foot thing?
MICHAEL SCHWAB: Weighs just under a ton.
AVIR: You gotta be kidding me.
MICHAEL SCHWAB: Mm-mm.
AVIR: Do you mind if I try to lift it?
MICHAEL SCHWAB: Absolutely.
AVIR: All right, here, hold—here, let's put this down here. Hold this and point it at me. All right. Okay, I do—I have been doing squats and deadlifts, so ...
MICHAEL SCHWAB: Good luck.
AVIR: I just want to give you a heads-up that I may be the one.
MICHAEL SCHWAB: Good luck.
AVIR: All right, so I'm just gonna put my arms around this thing.
MICHAEL SCHWAB: Okay.
AVIR: [grunts]
MICHAEL SCHWAB: It's a very futile effort, folks. It's just not happening.
AVIR: [grunts] Holy crap. I can't even budge it.
MICHAEL SCHWAB: No, you can't. But ...
AVIR: Can we try together? No? All right. All right.
LATIF: Okay, so now how is this gonna help Pat determine the age of the Earth?
AVIR: Well, he knows that this rock formed at the same time as the Earth. It's been perfectly preserved in space and ...
[clanking sound]
AVIR: Oh my God, it sings!
MICHAEL SCHWAB: Yeah.
AVIR: Whoa!
AVIR: … it's metal.
[clanking sound]
AVIR: This is a solid piece of metal.
MICHAEL SCHWAB: Yep. 92 percent iron and 7 percent nickel. And then that last one percent is 80 other trace elements.
AVIR: Including lead.
LATIF: Ah! Ba-da-bing, ba-da-boom, you just measure that thing with that thing, and then you got the age of the Earth.
AVIR: Kinda. I mean, like, look, these are tricky equations, tricky tools. It's not easy, so before you start measuring the meteorite you want to just—he just kind of wants to go practice on regular rocks, like regular granite rocks, just make sure that, you know, his technique is down.
LATIF: Got it. Because you have to measure it so precisely.
AVIR: It's gotta be so accurate. You really don't want to be making mistakes. So Pat takes a piece of granite, and the machine spits out these results that are totally confusing.
LATIF: Hmm.
AVIR: Like, his numbers are way off. There's way too much lead in the rock.
[ARCHIVE CLIP, Clair Patterson: Yeah, there was lead there that didn't belong there.]
AVIR: Like, this rock would have been formed a gabillion years ago.
LYDIA DENWORTH: And he's just saying, there's way too much lead, there's way too much lead. And he couldn't figure out ...
[ARCHIVE CLIP, Clair Patterson: Why?]
AVIR: But one day he's like, let me—you know what? Let me just run a blank sample.
LYDIA DENWORTH: Something that chemists do when they want to just test their system is you just run it ...
AVIR: Like, instead of putting this piece of rock in here, let me just run a blank.
LYDIA DENWORTH: And so when he did that, he still got, like, a whole lot of lead.
AVIR: So he's like "Okay, my blanks are not blanks."
LYDIA DENWORTH: So he knew that it was coming from the laboratory.
AVIR: This is a contamination issue.
LATIF: Right.
[ARCHIVE CLIP, Clair Patterson: Where did it come from?]
LYDIA DENWORTH: Okay.
[ARCHIVE CLIP, Clair Patterson: You go back and you track it down and say, "Well, it must be that."]
LYDIA DENWORTH: So first he started with glass beakers he was using.
AVIR: The vials are the first thing you're gonna look at. So he tests the vials. And he goes, "Shit."
[ARCHIVE CLIP, Clair Patterson: Lead.]
AVIR: These glass vials are made with lead, so let's get some new vials.
LATIF: Right.
AVIR: So gets new glass vials. Special ordered, never made with lead. Runs a sample again. It's still off.
LATIF: Huh.
AVIR: And so then he's like, "You know what? In the sample where I put the granite, I also put some water in it." And he realizes, actually, the water is coming from lead pipes.
LATIF: Ah.
AVIR: And so he's like, "Oh, crap. That's the problem." So he has to triple distill the water, boil it off, make sure he catches it in a vial that has no lead in it, to make sure that his water doesn't have any contamination from the pipes it came through. So he runs the sample and it's a little better, but there's still lead there. So now he's, like, obsessed. And Patterson, he's working in this lab.
LYDIA DENWORTH: And it was pretty grubby.
AVIR: He looks at the walls and he's like ...
LYDIA DENWORTH: There is peeling paint.
AVIR: So he tests the paint.
LYDIA DENWORTH: It was in the paint.
AVIR: So they repaint the walls. But still ...
LYDIA DENWORTH: There was way too much lead.
AVIR: Then he looks at his desk where the mass spectrometer is sitting on, and he figures out every joint in the desk is soldered together with lead.
LATIF: Oh, man!
AVIR: So he needs a new desk, new chairs with no lead. And then he uses Saran Wrap to cover every desk and every chair and every object in the room.
LATIF: [laughs]
AVIR: And still, too much lead.
LATIF: Wow!
AVIR: And so he thinks maybe there's some lead in the dust on the floors. So he starts mopping the floors. He gets the lead numbers to come down a little bit. And then one day, he notices a co-worker's lipstick is messing up his samples.
LATIF: Hmm.
AVIR: So he tests the makeup and he's like, "Okay, there's lead in there too."
LATIF: Wow.
AVIR: We can't wear makeup in this lab. And he eventually—he starts to get the lead number lower and lower. But then one day, he's working in the lab, and a little piece of his hair falls onto the desk, and the lead numbers shoot up.
LYDIA DENWORTH: He said, you know, "Holy shit!"
[ARCHIVE CLIP, Clair Patterson: Your hair!]
LYDIA DENWORTH: It's on him.
LATIF: [gasps] Wow! He's the contamination himself!
[ARCHIVE CLIP, Clair Patterson: The lead from your hair will contaminate the whole damn laboratory. Just from your hair. [laughs]]
AVIR: And so he shaves his head.
LATIF: [laughs]
AVIR: But then one day he decides, "Okay, well I'm just gonna test my skin." And he ends up seeing that there's a bunch of lead in his skin.
LATIF: Oh, no!
AVIR: It's everywhere.
LYDIA DENWORTH: There was lead in absolutely everything. And in the end, he made people—they had a little anteroom and you had to—you literally had to strip down to your underwear and put on this Tyvek suit.
AVIR: Which gets washed in acid.
LYDIA DENWORTH: And have little booties on and put plastic over their hair.
AVIR: He builds positive pressure air vents so the air is constantly blowing and pushing anything inside the lab outside of the lab. So even if you walk in with a little microgram of lead, the air may push it out.
LATIF: Hmm.
AVIR: He basically invents what we now call a "clean lab."
LATIF: Hmm.
AVIR: But he ultimately gets his samples down, his blank samples down to 0.1 micrograms. So that's one tenth of one millionth of a gram.
LATIF: Oof!
AVIR: And that took years.
LATIF: But is that still too much?
AVIR: No, that's fine. And so after this, he's finally actually ready to go to Canyon Diablo, get this meteorite and actually measure the damn thing that he was trying to do from the beginning.
LYDIA DENWORTH: And so he took these precious samples ...
AVIR: He puts the sample into the mass spec.
LYDIA DENWORTH: ... and it was late at night, he was there by himself.
AVIR: Just basically turns the crank and boom, pops out a number.
LYDIA DENWORTH: Which was four-and-a-half billion years.
LATIF: And that's—is that—is that, like, way ...
AVIR: That number is the age of the Earth.
LATIF: Wow!
AVIR: It's just a number, but what it represents, it's this fundamental truth. And in this moment, he's the only human being who knows the truth. He now has this window to that moment: the formation of not just the Earth, but the entire solar system. Sun, the planets, the moons, the rings around Saturn, me, you, everything comes from that.
LYDIA DENWORTH: I mean, it was so exciting for him. He thought he was having a heart attack. He went to visit his parents in Iowa the next day and he made them take him to the hospital, but he was fine. It was more like a lot of adrenaline coursing through him.
AVIR: He calms himself down, and he’s like, "Here I go, I got the number." He publishes it in a journal. And it's just—yawns.
LATIF: [laughs]
AVIR: Like, nobody cares.
[ARCHIVE CLIP, Clair Patterson: No one cared about—I mean, who cares? Even today, people don't care how old the Earth is.]
AVIR: So it definitely didn't get into any textbooks, it wasn't in the press. Seven years of his life has gone by.
LATIF: So it wasn't duck soup?
AVIR: It was not.
LATIF: What's a hard soup to make?
AVIR: It was like foie gras or something, I don't know.
LATIF: Yeah, okay. That’s good because it's also duck related, I feel like.
AVIR: Yeah, yeah.
LATIF: Okay.
[ARCHIVE CLIP, Clair Patterson: But I discovered in all this work, a story related to lead.]
AVIR: Like, this process of figuring out the age of the Earth sort of unveiled this truth about the world to him, which is that we're totally contaminated with lead.
LATIF: Right.
AVIR: I'm contaminated, you are contaminated. Every living thing is just filled with lead. And now that he's seen that, he—like, he can't turn away from that vision. Like, I almost think of it like The Matrix, you know? When you go into the matrix and you see those little green numbers falling or whatever and you just see the world for what it is, which is just like this thing that no one else is seeing, you can't look away.
LATIF: But what happens when no one believes what you're seeing? Back in a minute.
[LISTENER: This is Brie calling from Austin, Texas. Radiolab is supported in part by the Alfred P. Sloan Foundation, enhancing public understanding of science and technology in the modern world. More information about Sloan at www.sloan.org.]
[JAD ABUMRAD: Science reporting on Radiolab is supported in part by Science Sandbox, a Simons Foundation initiative dedicated to engaging everyone with the process of science.]
LATIF: Latif. Radiolab. Back with Avir, who started with a story about uranium, which has by now decayed into lead.
AVIR: Yeah, which I think is probably a boring topic. It's like talking about The Beatles, you know what I mean? It's just like everyone kind of knows about The Beatles. And lead. You know, you could talk to anybody, everybody now knows, like, oh, lead is bad for you. You know, lead in paint chips, like, don't eat lead. But, you know, back in the '50s, people really just didn't care about this. They didn't—they weren't thinking about lead. And science of the day said sure, lead poisoning is a thing. If you work in a mine and you, you know, don't wash your hands and then you eat a burger after touching lead ...
LATIF: Right.
AVIR: Then sure, you'll get lead poisoning. We've known that for thousands of years. But it's not like we're working in a lead mine, you know?
LATIF: Right.
AVIR: But for our dude Pat, he's like, "No, no, no. Come on. Like, something is going on here." And he's like, the only one who sees it.
[ARCHIVE CLIP, The Matrix: The matrix is everywhere.]
AVIR: That we're living in a dream. Like, we're living in a lead mine.
[ARCHIVE CLIP, The Matrix: You have to see it for yourself.]
AVIR: Okay, so Pat's finding all this lead in his lab. He's stripping down to his underwear, shaving his head. And he's telling people, and they're just like, "Dude, who cares? Like, it's just your lab."
LATIF: I definitely would have said that. I would have been like, "Okay. Yeah. So it's like an experimental nuisance, like that—I get that. You've proved that."
AVIR: Right. But Patterson believes no, this has to be bigger. Which leads him to this question, which is, okay, there's a lot of lead in my lab, but is there a lot of lead out in the world?
LYDIA DENWORTH: So ...
AVIR: Lydia Denworth again.
LYDIA DENWORTH: He started by looking in the ocean.
AVIR: So first thing he does is he gets a boat.
[ARCHIVE CLIP, interviewer: You weren't on the boat yourself, were you?]
[ARCHIVE CLIP, Clair Patterson: Yes, I was!]
[ARCHIVE CLIP, interviewer: Oh, you were on the boat. Okay. Okay.]
[ARCHIVE CLIP, Clair Patterson: And I got sick as a dog.]
AVIR: He has a big problem with sea sickness but he's just like, whatever.
[ARCHIVE CLIP, Clair Patterson: I hated it.]
AVIR: He goes out, collects a bunch of water from the Pacific Ocean. Takes the water back, analyzes it. And sure enough ...
[ARCHIVE CLIP, Clair Patterson: Lead!]
AVIR: There is a ton of lead in the ocean.
[ARCHIVE CLIP, Clair Patterson: So I said, okay.]
AVIR: Proof!
[ARCHIVE CLIP, Clair Patterson: So I published a paper.]
AVIR: And did it make waves in science or the public?
[ARCHIVE CLIP, Clair Patterson: No!]
AVIR: A lot of scientists were like ...
[ARCHIVE CLIP, Claire Patterson: They didn't care at all.]
AVIR: ... "If you say so. I don't really know. I also don't really care, you know?" But he's more convinced than ever. I mean, he starts walking around campus wearing a gas mask.
LYDIA DENWORTH: And a lot of people thought he was just plain crazy, right?
AVIR: Everyone's like, dude, lead is natural, it's probably always been there. This isn't some new crazy thing. But Pat just doesn't believe it. There's no way it's been like this forever. And then one day he gets into a conversation with a friend who gives him this idea.
LYDIA DENWORTH: That the place to look to understand the amount of lead in the air in the past ...
AVIR: Is the permafrost of the snow in, like, Greenland or Antarctica.
[ARCHIVE CLIP, Clair Patterson: Because snow in the polar regions. It comes out of the air.]
LYDIA DENWORTH: And lead, if it was in the air ...
[ARCHIVE CLIP, Clair Patterson: Then lead's in the snowflakes.]
LYDIA DENWORTH: It would come down out of the air and settle into the snow, and it would never leave because that snow and ice didn't melt.]
AVIR: And then the next year there's another snowfall. It doesn't melt. And then the year after that, there's another snowfall that doesn't melt.
[ARCHIVE CLIP, Clair Patterson: Next year you have another one. Next year you have another one.]
AVIR: And the snow starts to, like, layer on top of each other almost like the rings of a tree. It just—it's just preserved there.
LATIF: Because it's permafrost. It's permanent frost. It's like it's permanently there. Right.
AVIR: Yeah. Permafrost. So now it's 1964. He goes out to Greenland.
LYDIA DENWORTH: And he took his 15-year-old son with him.
CLAIR CAMERON PATTERSON III: [laughs] Well, I thought it was pretty cool.
AVIR: This is Pat's son.
CLAIR CAMERON PATTERSON III: My name is the same as his. Clair Cameron Patterson III, actually.
AVIR: He goes by Cam.
CLAIR CAMERON PATTERSON III: Always have.
AVIR: Basically, you know, they fly out to Greenland to this military base called Camp Century.
CLAIR CAMERON PATTERSON III: When you arrive there in a helicopter, you don't see anything except antennas and a few trailers and oil barrels.
AVIR: And snow. Snow that goes three miles deep. So Pat and Cam put on all this gear.
CLAIR CAMERON PATTERSON III: Dressed up in plastic suits.
AVIR: Gloves.
CLAIR CAMERON PATTERSON III: And ...
AVIR: Went down into this tunnel.
CLAIR CAMERON PATTERSON III: That went down into the ice.
[ARCHIVE CLIP, Clair Patterson: You go back in time.]
AVIR: And they cut out, like, four-by-four chunks of ice from the wall.
CLAIR CAMERON PATTERSON III: We had a melting trailer up on the surface.
AVIR: So they melt down this ice, ship it all the way back to the lab. And this is where Pat can really start to see the history of lead over time. And what he finds is that starting in, like, 1700, that there was basically no lead at all. Like, nothing. And then in, like, 1750, with the start of the Industrial Revolution, you see the lead levels start to go up and up and up and up.
LATIF: Hmm.
AVIR: Until 1930 ...
[ARCHIVE CLIP, Clair Patterson: Blam!]
AVIR: ... the lead levels skyrocket straight upward. And Patterson's like ...
[ARCHIVE CLIP, Clair Patterson: Well, hmm.]
AVIR: What the hell happened in 1930?
LYDIA DENWORTH: Well, The thing that changed was ...
[ARCHIVE CLIP, Clair Patterson: Leaded gasoline.]
AVIR: Suddenly ...
LYDIA DENWORTH: There was leaded gasoline in the air. So ...
AVIR: Long story short ...
LYDIA DENWORTH: In 1921...
AVIR: Car makers were trying to figure out how to get rid of something called engine knock.
LATIF: Engine knock.
AVIR: Knock. So like [knock knock knock].
LYDIA DENWORTH: Which is the pinging and bucking that engines do, and it slows down their efficiency.
AVIR: And so there were chemists trying all sorts of different things. And eventually they put a teaspoon of lead into some gasoline.
LYDIA DENWORTH: And the engine knock stopped immediately.
AVIR: Instantly gone. And people loved it. People wanted it. By 1960, 90 percent of cars were using leaded gasoline. And Pat's sort of looking at his results from this permafrost, and he's like ...
LYDIA DENWORTH: Holy smokes!
LATIF: This can't be good.
AVIR: Yeah.
LYDIA DENWORTH: It's everywhere. And he wrote this paper that basically said that lead had contaminated everything in the Earth, and that it's coming from leaded gasoline.
AVIR: And this time when his paper goes out into the world ... no one cared.
LYDIA DENWORTH: [laughs] Nope.
CLAIR CAMERON PATTERSON III: You know, he sounded like a crackpot.
AVIR: Cam says around this time at home, his dad would sort of lose it.
CLAIR CAMERON PATTERSON III: Get all excited, and ...
[ARCHIVE CLIP, Clair Patterson: Now these are chimpanzee idiots.]
CLAIR CAMERON PATTERSON III: Talk about the idiots that didn't understand anything.
[ARCHIVE CLIP, Clair Patterson: They're wrong, okay? And I knew that, okay? Now, ugh!]
AVIR: Because there were a lot of people being like, okay, sure, there's more lead in the air, but that doesn't mean there's more lead in us. Next, he basically gets a hold of 2,000-year-old ancient Peruvian skeletons.
LATIF: Mm-hmm.
AVIR: And takes their teeth, grinds up their teeth and measures the amount of lead in their teeth.
LATIF: Okay.
AVIR: Then he compares that to his own children's primary teeth.
LATIF: Oh, funny. It's like baby teeth. Yeah.
AVIR: Their baby teeth, yeah. He actually finds his children's teeth have 3,000 times more lead than the Peruvians.
LATIF: Oh God, that's terrible.
AVIR: And still, people don't care.
LATIF: Whoa!
AVIR: Because they're like, "Okay, there's more of it in us. Seems fine. It's not making you sick."
LYDIA DENWORTH: And when they say "making you sick," they meant, you know, killing you, making you blind, making you have seizures, things like that, right? Putting you in a coma.
AVIR: I mean, you know, it's not uranium, you know what I mean? It's not like people's hair and skin is just falling off and they're just vomiting blood, you know?
LATIF: Right.
AVIR: So ...
LATIF: It's more subtle than that.
AVIR: Maybe more subtle. That's the question, you know? Is this harmful?
LATIF: Right.
AVIR: And ...
LATIF: And what is the harm?
AVIR: And what is the harm, right? But what I think is interesting is, Pat doesn’t try to answer that question.
LATIF: Huh.
[ARCHIVE CLIP, interviewer: What was your motivation at this point? Were you thinking in an environmental sense?]
[ARCHIVE CLIP, Clair Patterson: No, I was not. Science, science, science. I didn't care two hoots about verifying what the contamination was.]
[ARCHIVE CLIP, interviewer: I see. So you were not being driven by environmental concerns?]
[ARCHIVE CLIP, Clair Patterson: I was not.]
AVIR: To be clear, Pat was convinced that lead was harmful, he just wasn't interested in doing the research that showed exactly how or to what extent or how to fix it.
[ARCHIVE CLIP, Clair Patterson: But I had friends and colleagues who were concerned about the environment. They were concerned about people being hurt by all this.]
AVIR: And Patterson would share his data with them.
[ARCHIVE CLIP, Clair Patterson: They'd come to my laboratory.]
AVIR: He would show them his techniques. And a lot of these people would go on to use this stuff to show just how harmful lead was.
[ARCHIVE CLIP, Clair Patterson: It was crucial in getting lead out of food can solder, getting lead out of glazes and this sort of stuff.]
AVIR: And while he knew that this was a good thing, to him it was like this slippery slope that led back into this messy world of human motivations, and into policy and politics.
LYDIA DENWORTH: And the thing was Patterson was so difficult to work with that he often didn't get invited to be on the national committees that were deciding things.
AVIR: And one of the few times he did, he shows up in DC and they're talking about, "What should we—what should we make recommendations to reduce the level of acceptable lead in the environment to?" And he just says it should be zero. That's the acceptable level. And so they—they do this without him. He refuses to sign the final report, and he writes at the bottom, "Dr. Patterson does not wish to be associated with this report." He writes his own addendum. He just writes this line: "The mining and smelting of lead within the human environment is actually a monumental crime committed by humanity against itself." Period.
[ARCHIVE CLIP, Clair Patterson: And it's oozing lead all over.]
AVIR: Regardless, the EPA did slowly start catching up to the science and banning lead from things.
[ARCHIVE CLIP, Clair Patterson: Actually now, they're getting lead out of paint.]
AVIR: But to Pat, that wasn't the purpose of science, not like real science. For him, science was how it was when he was a kid, you know? Where science isn't about solving some problem.
[ARCHIVE CLIP, Clair Patterson: It's not, "How can I solve this challenge?" But, "Why is that? Why is a drop of water spherical?" You know? ]
AVIR: And now that Pat had been able to prove what the natural level of lead should be, he was really just left with this simple question ...
[ARCHIVE CLIP, Clair Patterson: Why? Why? I mean, what led us to poison the Earth's biosphere with lead?]
AVIR: He started looking at ice cores that went all the way back to 800 BC, and what he found was this wasn't the first time that this had even happened, you know? Like, Greeks, Romans, every empire has poisoned themselves with lead.
[ARCHIVE CLIP, Clair Patterson: That proves for 2,000 years we have been unable to understand the evil that we are doing to ourselves.]
AVIR: And at this point, Patterson's question starts to shift. It becomes just a question of why are we this way?
[ARCHIVE CLIP, Clair Patterson: Okay? How—how do we think?]
AVIR: He actually starts writing a book, and it's ...
[ARCHIVE CLIP, Clair Patterson: This new concept of human consciousness.]
AVIR: ... all over the place. It's about ...
[ARCHIVE CLIP, Clair Patterson: Within the brain ...]
AVIR: ... neural pathways ...
[ARCHIVE CLIP, Clair Patterson: ... neuronal circuitries used ...]
AVIR: ... it's about ...
[ARCHIVE CLIP, Clair Patterson: ...abstract rationalization thinking ...]
AVIR: ... problem-solving ...
[ARCHIVE CLIP, Clair Patterson: ... formulation of religious myths.]
AVIR: ... human civilization, the nature of scientific thought.
CLAIR CAMERON PATTERSON III: He worked on this idea for years, and never really seemed to get anywhere. And it was tough on him.
AVIR: Cam said over time, he noticed his dad grew angrier, more jaded, and talked less, spent less time with his kids. And on December 5, 1995, he has an asthma attack in his office and he dies. He was 73.
AVIR: Less than a month after his death, January 1, 1996, the EPA banned the greatest source of all lead on Earth, the one that had driven Pat crazy—leaded gasoline. And obviously, lead is still with us today. Like, that's very clear, but in the years since Patterson's death, the amount of lead on the average freeway has decreased by 97 percent. And the average lead in people's blood in America has decreased by 94 percent. And lead experts say that all of that resulted in a five point increase in preschoolers' IQ. And just as a side note, the age of the Earth still stands at 4.5 billion years old, which is exactly as Patterson had calculated 70 years ago.
AVIR: But what really struck me when reporting this story is, like, how little recognition Clair Patterson got over the course of his life. But eventually, some of his students banded together to nominate him for the Tyler Prize, which is an environmental award. It's kind of a big deal. And he actually won the award eight months before his death. But by all accounts—including his own—he took no pride in it.
[ARCHIVE CLIP, Clair Patterson: I'm sorry to say I don't want it.]
[ARCHIVE CLIP, interviewer: Okay, okay.]
[ARCHIVE CLIP, Clair Patterson: I have—I have zero pride in any award, okay?]
[ARCHIVE CLIP, interviewer: Because I want to use the word "Pride." Can I use the word "Pleasure?" "Gratification?" Can I use any of these words?]
[ARCHIVE CLIP, Clair Patterson: No, not—gratification.]
[ARCHIVE CLIP, interviewer: "Pleasure." We have to use pleasure.]
[ARCHIVE CLIP, Clair Patterson: I—no. Good heavens. My whole—look, I'm stupid, all right? I'm not some brilliant person or whatever. I'm a—I'm a little child. I'm not smart. I mean, good scientists are like—they have the minds of children to see through all this facade of all this other stuff that they know is stupid nonsense. They don't notice it. They just don't see it the way that other people see it. Okay, so I'm not smart. So anyway, so these—it's only circumstantial. It's not—that's why I don't feel any honor. I'm not to be—it's not there. I'm not qualified to be honored. I mean, I'm—I'm just simply—it's accidental.]
LATIF: Reporter Avir Mitra. This episode was produced over millions of years by Matt Kielty along with Becca Bressler, Rachael Cusick and Maria Paz Gutiérrez. Special thanks to Cliff Davidson, Paul M. Sutter, Denton Ebel and Sam Keane. I'm Latif Nasser. Thanks for listening.
[LISTENER: Radiolab was created by Jad Abumrad and is edited by Soren Wheeler. Lulu Miller and Latif Nasser are our co-hosts. Suzie Lechtenberg is our executive producer. Dylan Keefe is our director of sound design. Our staff includes: Simon Adler, Jeremy Bloom, Becca Bressler, Rachael Cusick, W. Harry Fortuna, David Gebel, Maria Paz Gutiérrez, Sindhu Gnanasambandan, Matt Kielty, Annie McEwen, Alex Neason, Sarah Qari, Arianne Wack, Pat Walters, and Molly Webster. With help from Shima Oliaee, Sarah Sandbach, and Candice Wang. Our fact-checkers are Diane Kelly and Emily Krieger.]
-30-
Copyright © 2023 New York Public Radio. All rights reserved. Visit our website terms of use at www.wnyc.org for further information.
New York Public Radio transcripts are created on a rush deadline, often by contractors. This text may not be in its final form and may be updated or revised in the future. Accuracy and availability may vary. The authoritative record of programming is the audio record.